27 peer-reviewed scientific publications with more than 1000 citations and h-index (Google Scholar): 20 – Top Co-authors: Prof. Dimitrios N. Bikiaris (LINK) : [21] – Prof. George Z. Papageorgiou (LINK) : [20].
Published papers (27)
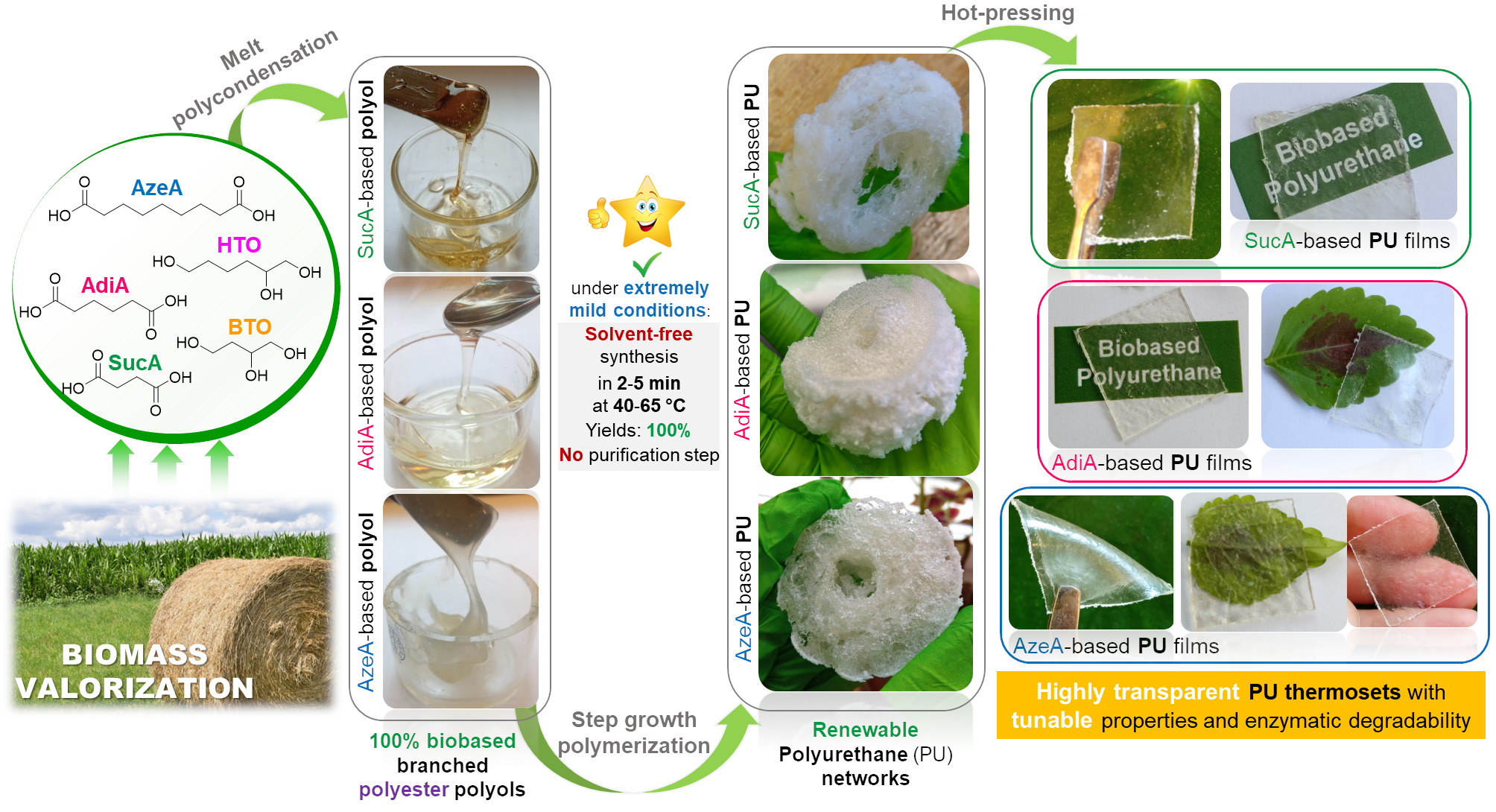
[27] N. Kasmi*, Y. Chebbi, A. Lorenzetti, M. Hakkarainen*. Highly transparent polyurethane thermosets with tunable properties and enzymatic degradability derived from polyols originating from hemicellulosic sugars, Green Chemistry 2023, 25, 9908-9925. LINK
Abstract: Polyols made entirely from biomass could play a substantial role in producing greener polyurethanes (PU) with lower environmental impacts. Here, six fully biobased branched polyester polyols derived from hemicellulosic sugars and dicarboxylic acids were prepared by melt polycondensation and further utilized for the synthesis of twelve highly transparent and malleable PU thermosets. The latter were prepared by solvent-free step growth polymerization under extremely mild reaction conditions, easily applicable industrially. This included a very short reaction time (125-335 s), low operating temperatures (40-65 °C) and excellent yields (100%). In addition, no purification step was required. All the obtained PUs possessed very good thermal stability exceeding 235 °C, Tg (3.6-70.4 °C), and a broad hot-pressing window up to 192 °C above respective Tg. These amorphous materials demonstrated a wide range of stress-strain behaviors, from hard to ductile, with elongation at break and tensile strength appearing in the 15-188% and 3.3-31.1 MPa range, respectively; comparable or superior to common commercially available fossil-based PU thermosets in the market. Enzymatic hydrolysis behaviour of the synthesized PUs was assessed using lipases from Candida rugosa and Aspergillus niger. All PUs showed some susceptibility to enzymatic attack, with a maximum mass loss up to 35% after 30 days. Most importantly, it was found that compositional control by tailoring of the length of the diacid unit in the branched polyols backbone and/or chemical crosslinking degree of the resulting PU networks can be used as a practical tool to effectively ‘on demand’ tune thermal properties, mechanical performance and enzymatic degradation rate. Taking advantage of these promising features in combination with the optical transparency, the developed polyurethane thermosets with easily adjustable properties show great potential as innovative materials for a wide applications range.
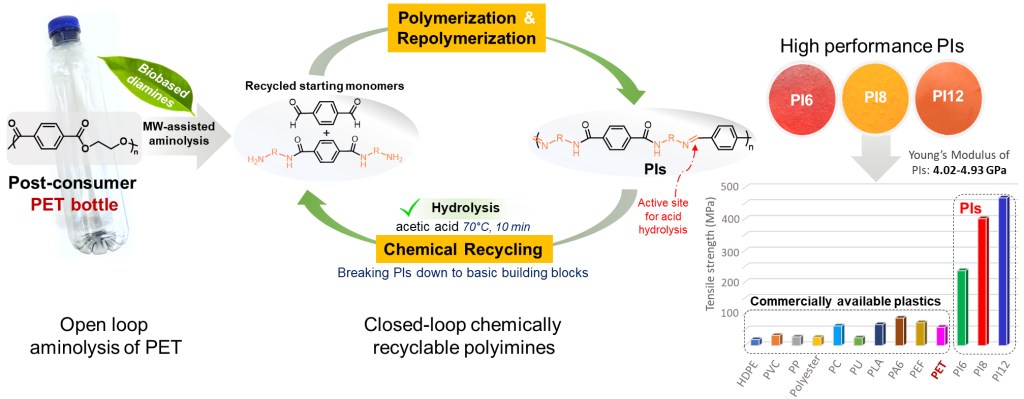
[26] N. Kasmi, E. Bäckström, M. Hakkarainen*. Open-loop recycling of post-consumer PET to closed-loop chemically recyclable high-performance polyimines, Resources, Conservation & Recycling 2023, 193, 106974 (IF: 13.716). LINK

Abstract: Intriguing high-performance polyimines (PI) were designed from diamines recovered by open-loop recycling of postconsumer PET bottles by microwave-assisted aminolysis. These new PIs had excellent thermal properties (Tm = 226–286 °C, Tg = 88–148 °C, heat resistance up to 327 °C) and super-high toughness and strength with Young’s modulus of 4.02–4.93 GPa and tensile strength of 237–467 MPa, both significantly higher compared with common engineering plastics. The synthesized PIs also demonstrated recyclability “on demand” to original building blocks via mild and ultrafast acetic acid catalysed hydrolysis (70 °C for 10 min). Furthermore, the recovered monomer mixture was directly repolymerizable providing attractive closed-loop polymer-to-polymer recyclability under extremely mild conditions. These high performance thermoplastics, with easily tunable properties by selection of diamine used for aminolysis, in combination with closed-loop chemical recyclability have great promise as next-generation circular materials, designed from recycled plastics waste, for a wide property and application range.
[25] M. Safari, N. Kasmi, C. Pisani, V. Berthé, A. J. Müller*, Y. Habibi. Effect of the structural features of linear bio-based polyester plasticizers on the crystallization of polylactides, International Journal of Biological Macromolecules 2022, 214, 128-139 (IF “2021”: 8.025). LINK

Abstract: This work presents, for the first time, a detailed report on how the nucleation and crystallization of polylactide (PLLA) are affected by biobased aliphatic polyesters plasticizers. Three biobased polyesters were synthesized via solvent-free two-stage melt polycondensation of adipic acid (AdA) with three different biobased aliphatic diols and used as plasticizers for poly(L-lactic acid) (PLLA). The molecular structure of the synthesized polyesters was proved using 1H NMR, 13C NMR and Fourier transform infrared (FTIR) spectroscopy. PLLA/AdA-based blends containing 10 wt % of the polyester plasticizers were studied by tensile tests, dynamic mechanical analysis (DMA), wide-angle x-ray scattering (WAXS), differential scanning calorimetry (DSC) and polarized light optical microscopy (PLOM). Adding the plasticizers to PLLA decreased Tg by up to 11 ºC and significantly increased the elongation at break by about 8 times compared with neat PLLA. The addition of 10 wt% of any AdA-based plasticizer to PLLA increases the nucleation rate from the glassy state by around 50-110% depending on the plasticizer. The overall crystallization rate from the glassy state was 2-3 times faster for the plasticizer PLLAs than neat PLLA. These results are a consequence of the lower energy barrier for both nucleation and growth processes. The incorporation of AdA-based linear polyesters had an incremental impact on the crystal growth rate (or secondary nucleation) of PLLA spherulites from the melt and glassy states. In conclusion, the AdA-based aliphatic polyesters allowed to enhance PLLA crystallization rates and showed interesting potential for the formulation of fully biobased PLLA blends.

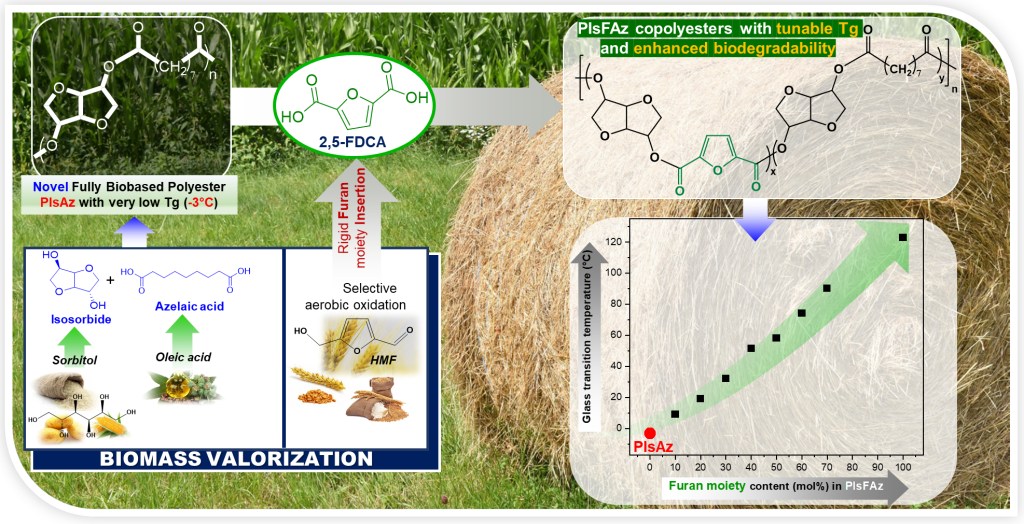
[24] N. Kasmi*, Z. Terzopoulou, Y. Chebbi, R. Dieden, Y. Habibi, D. N. Bikiaris. Tuning thermal properties and biodegradability of poly(isosorbide azelate) by compositional control through copolymerization with 2,5-furandicarboxylic acid. Polymer Degradation and Stability 2022, 195, 109804 (IF “2021”: 5.204) LINK

Abstract: A new fully biobased poly(isosorbide furanoate-co-azelate) (PISFAz) copolyesters series was synthesized through melt polycondensation. Incorporation of 2,5-furandicarboxylic acid (FDCA), a rigid comonomer, at different molar ratios in poly(isosorbide-azelate) homopolymer led to tuning of the thermal properties and biodegradability of the resulting copolyesters. The PISFAz copolyesters with various FDCA molar content spanning from 10 to 70% were prepared and characterized by FTIR, GPC, 1D/2D NMR and viscosity measurements. It was found that PISFAz were totally amorphous materials with high thermal stability. NMR results indicated that random microstructures were obtained for the prepared copolymers with high azelaic acid content (⩾ 60 mol%). Most notably, the inclusion of FDCA units into the copolymer molecular chains induced a significant increase in the glass transition temperatures (Tg) that varied from 9.2 to 91.1°C depending on FDCA content, leading to copolyesters with tunable Tg over a wide temperature window. The enzymatic hydrolysis behavior of PISFAz was assessed using lipases from Pseudomonas cepacia and Rhizopus oryzae revealing different susceptibility to enzymatic attack depending on the comonomer ratio, with a maximum degradation rate up to 61% after 30 days. These novel furanoate-based copolyesters show great potential to serve as promising green thermoplastic materials for applications requiring high Tg values.
[23] D. G. Papageorgiou*, I. Tsetsou, R. O. Ioannidis, G. Nikolaidis, S. Exarhopoulos, N. Kasmi, D. N. Bikiaris, D. Achilias, G. Z. Papageorgiou*. A new era in engineering plastics: compatibility and perspectives of sustainable alipharomatic poly(ethylene terephthalate)/poly(ethylene 2,5-furandicarboxylate) blends. Polymers 2021, 13(7), 1070 ( IF “2021”: 4.967) LINK

Abstract: The industrialisation of poly(ethylene 2,5-furandicarboxylate) for total replacement of poly(ethylene terephthalate) in the polyester market is under question. Preparation of high-performing polymer blends is a well-established strategy for tuning the properties of certain homopolymers and create tailor-made materials to meet the demands for a number of applications. In this work, the structure, thermal properties and the miscibility of a series of poly(ethylene terephthalate)/poly(ethylene 2,5-furandicarboxylate) (PET/PEF) blends have been studied. A number of thermal treatments were followed in order to examine the thermal transitions, their dynamic state and the miscibility characteristics for each blend composition. Based on their glass transition temperatures and melting behaviour the PET/PEF blends are miscible at high and low poly(ethylene terephthalate) (PET) contents, while partial miscibility was observed at intermediate compositions. The multiple melting was studied and their melting point depression was analysed with the Flory-Huggins theory. In an attempt to further improve miscibility, reactive blending was also investigated.
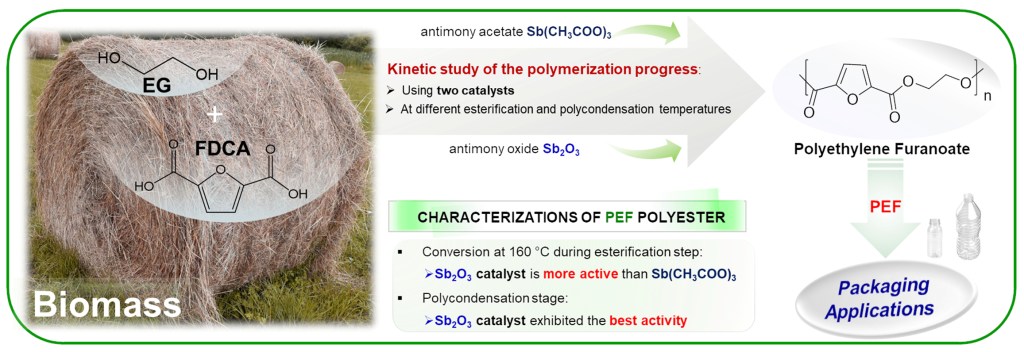
[22] L. Papadopoulos, A. Zamboulis, N. Kasmi, M. Wahbi, C. Nannou, D. A. Lambropoulou, M. Kostoglou, G. Z. Papageorgiou, D. N. Bikiaris*. Investigation of the catalytic activity and reaction kinetic modeling of two antimony catalysts in the synthesis of poly(ethylene furanoate). Green Chemistry 2021, 23, 2507-2524 (IF “2021”: 11.034) LINK
Abstract: In the last decades, the interest in the synthesis and development of novel biobased polymers with interesting properties, able to compete with the existing petroleum-based polymers has grown exponentially. 2,5-furandicarboxylic acid (FDCA) and poly(ethylene furanoate) (PEF) are the leaders of biobased aromatic polymers. However, the production of PEF by the direct esterification of FDCA with ethylene glycol (EG) has been shadowed by the transesterification of dimethyl 2,5-furandicarboxylate. Herein, we present the kinetic study of the polymerization progress of high-purity FDCA with EG using two different antimony catalysts (antimony oxide, Sb2O3, and antimony acetate, Sb(CH3COO)3) and different esterification and polycondensation temperatures by the traditional two-step polycondensation method. Each step was monitored by suitable characterization techniques, such as intrinsic viscosity measurements, carboxylic acid end-group analysis, nuclear magnetic resonance spectroscopy, infra-red spectroscopy and differential scanning calorimetry. Further insight into the esterification step, was given by high resolution mass spectrometry enabling the accurate mass measurement of the oligomers. Finally, theoretical studies were conducted to estimate the kinetics of the polymerization of PEF during esterification and polycondensation stages.
[21] N. Kasmi, C. Pinel, D. Da Silva Perey, R. Dieden, Y. Habibi. Synthesis and characterization of fully biobased polyesters with tunable branched architectures. Polymer Chemistry 2021, 12, 991-1001 (IF “2021”: 5.364). LINK

Abstract: A series of sugar-derived triols and biobased diacids were combined to prepare fully biobased branched polyesters with different structural features by melt polycondensation. By applying the BiMolecular Non-Linear Polymerization methodology (BMNLP), the molar ratio of diacid/triols was varied to access branched polyesters bearing either hydroxyl or carboxyl moieties as end groups. The structural features of the resulting polymers were scrutinized by 1H/13C NMR, and FT-IR spectroscopies, whereas DSC and TGA were used to investigate their thermal properties. The structure-property relationship of the synthesized polyesters was correlated to the structure of the triols and diacids and their molar ratio. Both prepared carboxyl-ended and hydroxyl-terminated branched polyesters were amorphous with relatively low glass transition temperatures ranging between -57 and -18 °C for hydroxyl terminated polyesters while for carboxyl terminated ones, they oscillate between -37 and 19 °C. All these polyesters exhibit good heat resistance with onset degradation temperature Td,5% ranging from 180 to 268 °C and from 168 to 236 °C for COOH- and OH-end groups-bearing polymers series, respectively. The structural features and properties of the resulting branched fully biobased polyesters make them not only potential candidates for a wide range of applications but also as intermediate substrates for further chemical modifications and/or chain extension to access a wide range of functional (co)polymer materials.

[20] N. Kasmi, L. Papadopoulos, Y. Chebbi, G.Z. Papageorgiou, D.N. Bikiaris*. Effective and facile solvent-free synthesis route to novel biobased monomers from vanillic acid: structure–thermal property relationships of sustainable polyesters. Polymer Degradation and Stability. 2020, 181, 109315. LINK (IF “2021”: 5.204)

Abstract: Solvent-free synthesis of monomers is one among the most promising ways to develop greener polymers that are both environmentally and economically acceptable, but it was described as one of the “grand challenges” facing chemists. As a contribution towards sustainable bioplastics development which has attracted great attention in materials science research, a truly efficient, practical, and more environmentally friendly solvent-free synthetic route was successfully applied herein to prepare three new fully biobased diol monomers derived from vanillic acid and aliphatic diols (ethylene glycol, 1,3-propanediol and 1,4-butanediol). Their chemical structures were confirmed in detail by 1H, 13C NMR and FTIR spectroscopies while their thermal properties were investigated by DSC and TGA. Results showed high melting points in the 121.8–142.3 °C range and no significant weight loss (Td, 5%) up to 243, 312 and 284 °C respectively for diols with 2, 3, and 4 methylene units. To prove their suitability in polymerization, melt polycondensation of prepared diols with three diacyl chlorides and also with dimethyl 2,5-furandicarboxylate were successfully carried out under catalyst-free conditions and using tetrabutyl titanate (TBT), respectively. The chemical structures of the novel series of polyesters were confirmed in detail by NMR and FTIR spectroscopies. The latter showed satisfactory intrinsic viscosity values in the 0.25–0.30 dL/g range and a wholly amorphous nature. All materials revealed high thermal stability with onset degradation temperatures Td, 5% ranging from 314 to 373 °C and a wide glass transition temperature (Tg) range oscillating from -2.8 to 69.5 °C. The innovative approach proposed herein for the first time, which involves the synthesis of sustainable monomers under solvent-free conditions, is fully aligned with one of the main principles of green chemistry.

[19] B. Quienne, N. Kasmi, R. Dieden, S. Caillol, Y. Habibi*. Isocyanate-free fully biobased star polyester-urethanes: synthesis and thermal properties. Biomacromolecules, 2020, 21, 5, 1943-1951. LINK (IF “2020”: 6.988)

Abstract: A green strategy for the synthesis of non-isocyanate polyester-urethanes (NIPHEUs) was developed. These NIPHEUs were synthesized by step growth polymerization combining sugar-derived dimethyl-2,5-furan dicarboxylate (DMFD) with polyhydroxylurethanes (PHUs) adducts bearing four hydroxyl groups. The later hydroxyl urethane tetraols (HU-tetraols) building blocks were prepared by aminolysis of glycerol carbonate with two different aliphatic diamines having different chain lengths, 8 and 12 carbons. Qualitative and quantitative NMR analyses of the HU-tetraols showed the presence of primary and secondary hydroxyl moieties at different ratios. Hence, in the polycondensation stage, the stoichiometry of diacids was varied from 1 to 6 equivalents in order to tailor the structural features of the prepared NIPHEUs. The success of the chain extension through polycondensation was confirmed by FTIR and NMR analyses. Thermal analyses of these new polymers demonstrated satisfactory thermal stability with onset degradation temperatures ranging from 170 to 220°C where the main first degradation stage occurs. Their melting temperatures ranged between 93 and 110°C and seem to be driven by the thermal behavior of HU-tetraol monomers. Surprisingly, preliminary results from thermal analyses revealed the occurrence of a striking thermal change in the NIPHEUs upon repetitive heating cycles. This behavior may be related to a thermal induced bond exchange probably driven by transcarbamoylation reaction. Such interesting behavior for this new type of NIPHEUs would be unique and should be confirmed by a deeper study before leading to a new range of functional green materials.

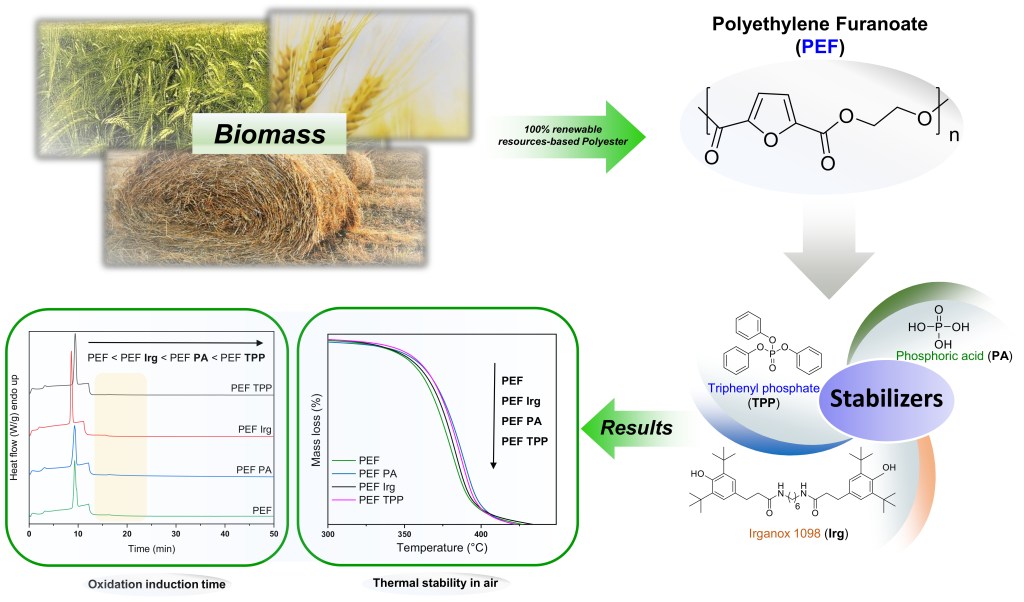
[18] Z. Terzopoulou, M. Wahbi, N. Kasmi, G.Z. Papageorgiou. D.N. Bikiaris*. Effect of additives on the thermal and thermo-oxidative stability of poly(ethylene furanoate) biobased polyester. Thermochimica Acta. 2020, 686, 178549. LINK (IF “2021”: 3.378)

Abstract: Polyesters based on 2,5-furan dicarboxylic acid are a new class of biobased polymers with properties superior of those of their fossil-based homologues. However, similarly to poly(ethylene terephthalate) (PET), poly(ethylene 2,5-furan dicarboxylate) (PEF) degrades during its thermal processing and as a result its molecular weight drops, and discoloration occurs. This is mainly attributed to thermal and thermo-oxidative degradation reactions. The addition of several types of thermal stabilizers during the synthesis or the processing of thermoplastic polyesters is a common practice. In this work, a commercial phenolic antioxidant (Irganox 1098), and two phosphorus-containing thermal stabilizers (phosphoric acid and triphenyl phosphate) were added in PEF polyester during synthesis with the use of antimony acetate catalyst. The effect of these additives on the molecular weight, thermal properties, thermal, thermo-oxidative stability and physical aging of PEF was evaluated. It was found that all additives slightly improved the thermal stability of PEF, with phosphoric acid and mainly triphenyl phosphate being the most efficient ones.
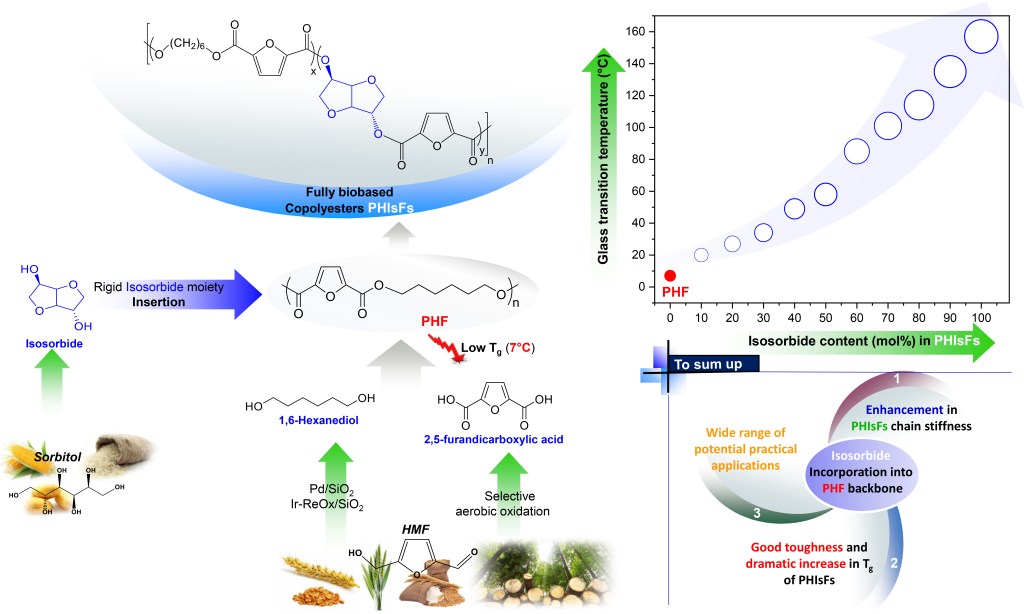
[17] N. Kasmi, N. Ainali, E. Agapiou, L. Papadopoulos, G.Z. Papageorgiou. D.N. Bikiaris*. Novel High Tg fully biobased poly(hexamethylene-co-isosorbide-2,5-furan dicarboxylate) Copolyesters: Synergistic Effect of Isosorbide Insertion on Thermal performance Enhancement. Polymer Degradation and Stability. 2019. LINK (IF “2021”: 5.204)

Abstract: Here an effective solution for overcoming the low glass transition temperature (Tg) of poly(hexamethylene 2,5-furan dicarboxylate) (PHF), a fully biobased polyester derived from dimethylfuran-2,5-furan-dicarboxylate (DMFD) and 1,6-hexanediol (1,6-HD), is proposed that uses isosorbide (Is) as a bicyclic rigid diol comonomer. Incorporating this sugar-derived diol with a broad scope content (3–90 mol%) into PHF macromolecular chain, by melt polycondensation and using titanium (IV) isopropoxide (TTIP) catalyst, results in the synthesis of highly heat-resistive poly(hexamethylene-co-isosorbide-2,5-furandicarboxylates) copolyesters (PHIsF). The chemical structure and composition of the prepared 100% renewable resources-based materials were confirmed in detail by 1H NMR and FTIR spectroscopies. Random microstructures were obtained for PHIsF samples, when Is content exceeds 40 mol%. As revealed by Wide-Angle X-ray Diffraction (WAXD) patterns, increase of IsF content in the copolymers leads to amorphous materials. The latter exhibited an excellent thermal stability up to 360 °C and very variable Tg values oscillating from 10 to 135 °C depending on the comonomer ratio, in which it gradually increases with increasing of Is feed content. Results found herewith showed that the high stiff building block Is can be used as an effective control parameter to spectacularly enhance the thermal properties of polymers, particularly the glass transition temperature. Taking advantage of their features, PHIsF have the potential to serve as promising fully biobased amorphous materials for practical applications that demand high Tg values.
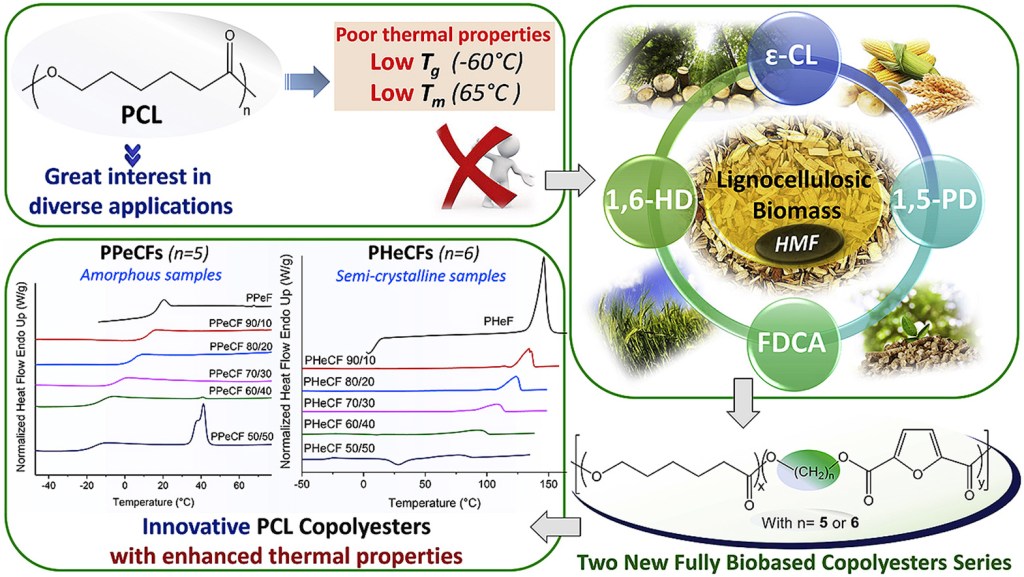
[16] N. Kasmi, M. Wahbi, L. Papadopoulos, Z. Terzopoulou, N. Guigo, N. Sbirrazzuoli, G.Z. Papageorgiou*. D.N. Bikiaris*. Synthesis and characterization of two new biobased poly(pentylene 2,5-furandicarboxylate-co-caprolactone) and poly(hexamethylene 2,5-furandicarboxylate-co-caprolactone) copolyesters with enhanced enzymatic hydrolysis properties. Polymer Degradation and Stability. 2019, 160, 242- 263. LINK (IF “2021”: 5.204)

Abstract: Herein, two fully renewable copolyester series, namely poly(pentylene 2,5-furandicarboxylate-co-caprolactone) (PPeCFs) and poly(hexamethylene 2,5-furandicarboxylate-co-caprolactone) (PHeCFs) were successfully synthesized with combining ε-caprolactone (CL) with poly(pentylene 2,5-furandicarboxylate) (PPeF) and poly(hexamethylene 2,5-furan dicarboxylate) (PHeF) with different molar ratios. These materials, with a CL content ranging from 10 to 50 mol%, were synthesized for first time using stannous octoate as catalyst via ring opening polymerization (ROP). Their chemical structures and molar composition were evaluated by 1H NMR, 13C NMR and FTIR spectroscopies, while their thermal properties were investigated in detail using Fast Scanning Calorimetry (FSC), Differential scanning calorimetry (DSC) and Thermogravimetric analysis (TGA). The obtained results, in combination with Wide-Angle-X-ray diffractometry (WAXD), showed that copolymerization of CL with PHeF and PPeF led to semi-crystalline and partially amorphous copolyesters respectively, providing the basis for significant thermal properties enhancement with respect to the polycaprolactone (PCL) homopolymer, and therefore a much wider range of melting points (Tm) and glass transition temperatures (Tg) were obtained. TGA of the new copolymers showed excellent thermal stability, exceeding 310 °C and 360 °C for PHeCFs and PPeCFs respectively, while their decomposition mechanism was evaluated by pyrolysis-gas chromatography/mass spectroscopy (Py-GC/MS). Almost all copolyesters and mainly the ones with 40 and 50 mol% CL content showed accelerated enzymatic hydrolysis rate.
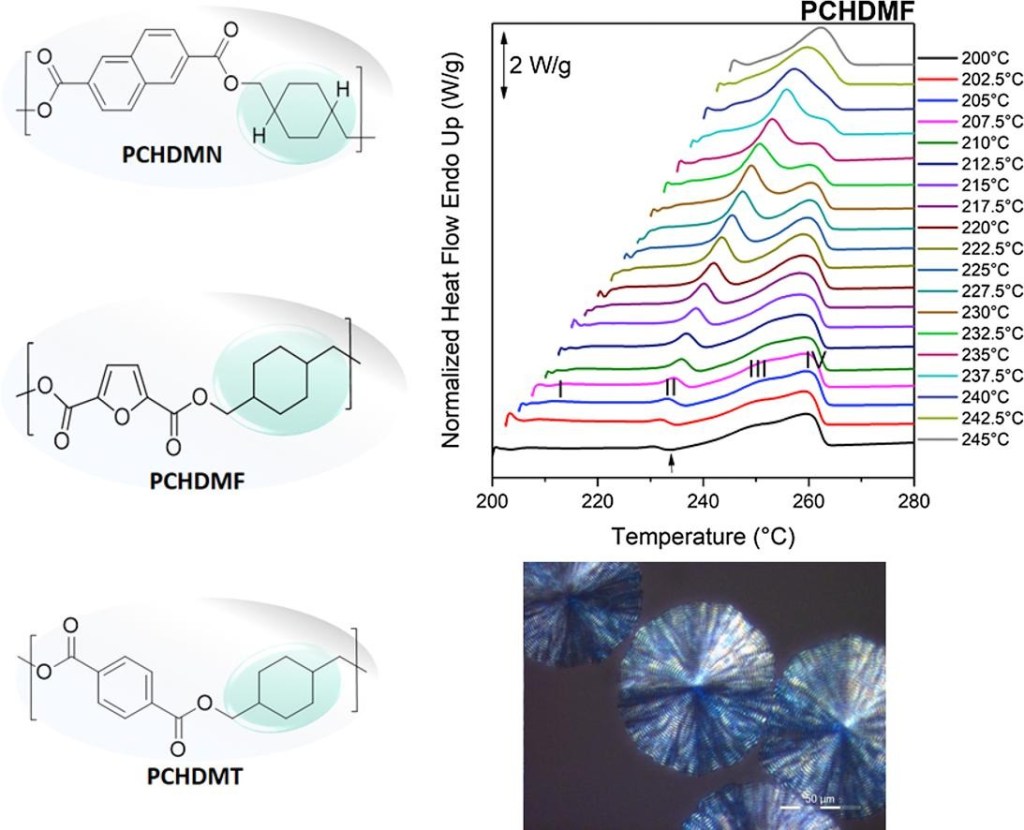
[15] N. Kasmi, N. Poulopoulou, Z. Terzopoulou, D.G. Papageorgiou*, D.N. Bikiaris, G.Z. Papageorgiou*. Sustainable Thermoplastics from Renewable Resources: Thermal behavior of Poly(1,4-cyclohexane dimethylene 2,5-furandicarboxylate), European Polymer Journal. 2019, 112, 1-14. LINK (IF “2021”: 5.546)

Abstract: Poly(1,4-cyclohexane dimethylene 2,5-furandicarboxylate) (PCHDMF) is a sustainable thermoplastic that can be prepared from renewable resources with potential uses as a replacement for its terephthalate (PCHDMT) and naphthalate (PCHDMN) analogues. A detailed study has been undertaken to assess its thermal behavior and chemical/structural characteristics, in comparison to its counterparts. The melting temperature of PCHDMF was observed at Tm = 264.5 °C, the glass transition was obtained at 77 °C and the cold crystallization temperature was seen at 121 °C. The melting of the polymers was studied under a variety of conditions and all samples displayed the characteristic melting-recrystallization-remelting behavior. Isothermal and dynamic crystallization tests revealed that PCHDMF crystallizes at faster rates than its analogues, while the equilibrium melting point of PCHDMF was established at 300 °C. The enthalpy of fusion values for the polyesters were found ΔHm = 137 J/g for PCHDMF, ΔHm = 108 J/g for PCHDMT, ΔHm = 119 J/g for PCHDMN. Using the Lauritzen-Hoffman analysis of spherulite growth rates, larger Kg values were found for PCHDMF, due to its less flexible structure.
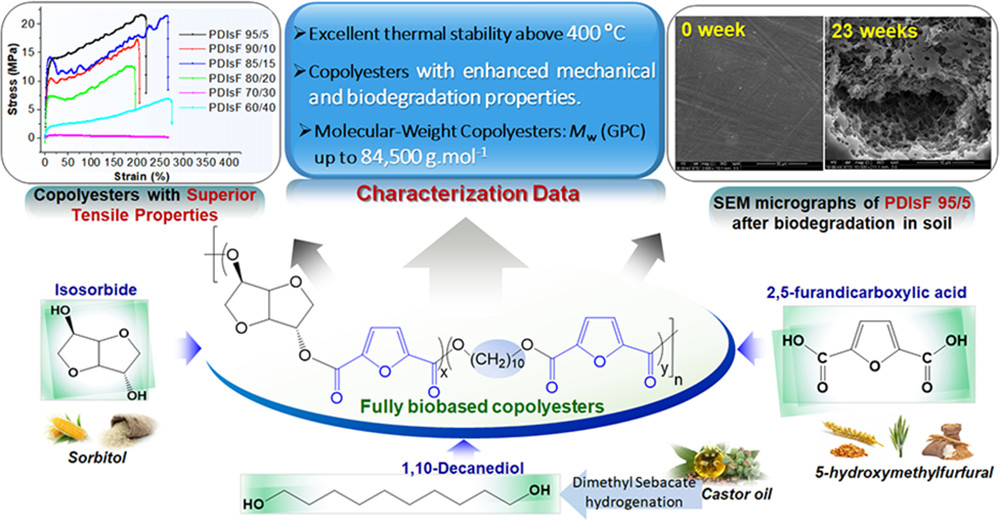
[14] Y. Chebbi, N. Kasmi, M. Majdoub, P. Cerruti, G. Scarinzi, M. Malinconico, G. Dal Poggetto, G.Z. Papageorgiou, D.N. Bikiaris*. Synthesis, Characterization, and Biodegradability of Novel Fully Biobased Poly(decamethylene-co-isosorbide 2,5-furandicarboxylate) Copolyesters with Enhanced Mechanical Properties. ACS Sustainable Chemistry & Engineering. 2019, 7 (5), 5501-5514. LINK (IF “2020”: 8.198)

Abstract: This study spotlighted a successful synthesis of a novel series of biobased poly(decamethylene-co-isosorbide 2,5-furandicarboxylate)s (PDIsFs) copolyesters from dimethylfuran-2,5-dicarboxylate (DMFD), isosorbide (Is), and 1,10-decanediol (1,10-DD) by melt polycondensation, using titanium(IV) isopropoxide (TTIP). The chemical structure and composition of prepared polymers were confirmed in detail by 1H NMR and FTIR spectroscopies. Satisfactory weight-average molecular weights (Mw) in the 55,300–84,500 g/mol range and random microstructures were obtained for PDIsFs. It was shown that Is unit incorporation into the copolyesters molecular chains was dramatically effective in increasing the glass transition temperatures (Tg) and in delaying the onset decomposition temperatures of PDIsFs. Hence, an excellent improvement of the thermal stability exceeding 405 °C for all copolymers was obtained. In addition, the degradation behavior in soil as well as the mechanical properties of PDIsFs were duly investigated in detail. The biodegradation rate of the copolyesters depended on the comonomer ratio. Rotational rheometry characterization of polymer melts revealed prevailing viscous properties for all formulations, whereas the presence of isosorbide favored a Newtonian behavior. Oxygen induction time (OIT) measurements by chemiluminescence (CL) demonstrated that isosorbide incorporation also dramatically increases polymer thermo-oxidative stability. Taking advantage of their features, PDIsFs have the potential to serve as promising and innovative biobased polymers for practical applications such as ecofriendly and sustainable plastic packaging.
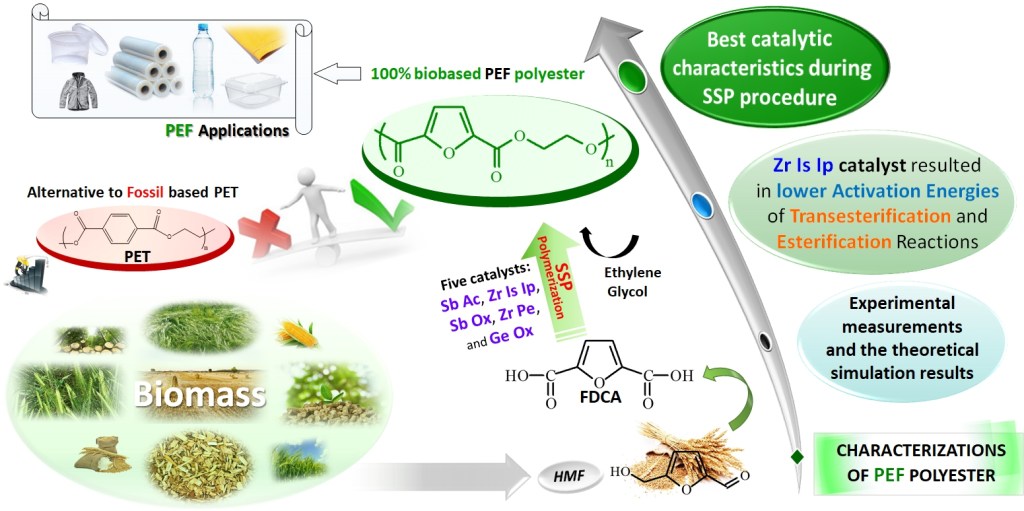
[13] Y. Chebbi, N. Kasmi, M. Majdoub, G.Z. Papageorgiou*, D.N. Achilias, D.N. Bikiaris*. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase. Polymers. 2019, 11(3), 438. LINK (IF “2021”: 4.967)

Abstract: In this study, the synthesis of poly(ethylene furanoate) (PEF), catalyzed by five different catalysts—antimony acetate (III) (Sb Ac), zirconium (IV) isopropoxide isopropanal (Zr Is Ip), antimony (III) oxide (Sb Ox), zirconium (IV) 2,4-pentanedionate (Zr Pe) and germanium (IV) oxide (Ge Ox)—via an industrially common combination of melt polymerization and subsequent solid-state polymerization (SSP) is presented. In all reactions, proper amounts of 2,5-dimethylfuran-dicarboxylate (DMFD) and ethylene glycol (EG) in a molar ratio of DMFD/EG= 1/2 and 400 ppm of catalyst were used. Polyester samples were subjected to SSP procedure, under vacuum application, at different reaction times (1, 2, 3.5, and 5 h) and temperatures of 190, 200, and 205 °C. Carboxyl end-groups concentration (–COOH), intrinsic viscosity (IV), and thermal properties, via differential scanning calorimetry (DSC), were measured for all resultant polymers to study the effect of the used catalysts on the molecular weight increase of PEF during SSP process. As was expected, it was found that with increasing the SSP time and temperature, the intrinsic viscosity and the average molecular weight of PEF steadily increased. In contrast, the number of carboxyl end-groups content showed the opposite trend as intrinsic viscosity, that is, gradually decreasing during SSP time and temperature increase. It is worthy to note that thanks to the SSP process an obvious and continuous enhancement in the thermal properties of the prepared PEF samples was attained, in which their melting temperatures (Tm) and degree of crystallinity (Xc) increase progressively with increasing of reaction time and temperature. To predict the time evolution of polymers IV, as well as the hydroxyl and carboxyl content of PEF polyesters during the SSP, a simple kinetic model was developed. From both the theoretical simulation results and the experimental measurements, it was demonstrated that surely the Zr Is Ip catalyst shows the best catalytic characteristics compared to all other used catalysts herein, that is, leading in reducing—in a spectacular way—the activation energy of the involved both transesterification and esterification reactions during SSP.
[12] N. Poulopoulou, A. Pipertzis, N. Kasmi, D.N. Bikiaris, D.G. Papageorgiou, G. Floudas, G.Z. Papageorgiou*. Green polymeric materials: On the dynamic homogeneity and miscibility offuran-based polyester blends. Polymer. 2019, 174, 187-199. LINK (IF “2021”: 4.432)

Abstract: Polyesters from 2,5-furandicarboxylic acid are considered as biobased alternatives to their terephthalate counterparts. This work is focused on polymer blends comprised of furan-based polyesters differing by one or two methylene groups in their repeat units. Blends of Poly(ethylene 2,5-furandicarboxylate) (PEF) and poly(propylene 2,5-furandicarboxylate) (PPF) displayed a single composition-dependent glass temperature (Tg). Dielectric spectroscopy results revealed dynamically homogeneous mixtures when the backbones differ by a single methylene unit and dynamically heterogeneous mixtures when they differ by two methylene units. The most interesting finding was the single segmental dynamics in the PEF-PPF and poly(butylene furanoate)-poly(propylene furanoate) (PBF-PPF) blends. For comparison, homologous terephthalate blends were also investigated. Finally, reactive blending was applied in PEF-PBF blends and enhanced compatibility was achieved even after short times due to transesterification reactions at elevated temperatures. With increasing duration of melt-mixing the thermodynamic miscibility between PEF and PBF increased and ultimately a random copolymer was obtained.
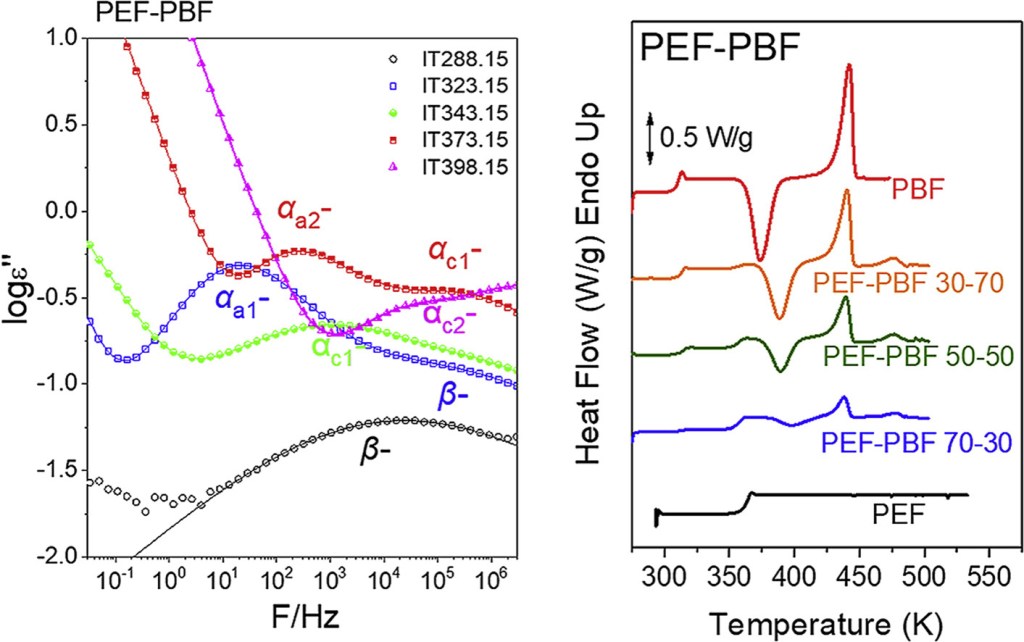
[11] N. Poulopoulou, N. Kasmi, M. Siampani, Z.N. Terzopoulou, D.N. Bikiaris, D.S. Achilias, D.G. Papageorgiou*, G.Z. Papageorgiou*. Exploring Next-Generation Engineering Bioplastics: Poly(alkylene furanoate)/Poly(alkylene terephthalate) (PAF/PAT) Blends. Polymers. 2019, 11(3), 556. LINK (IF “2021”: 4.967)

Abstract: Polymers from renewable resources and especially strong engineering partially aromatic biobased polyesters are of special importance for the evolution of bioeconomy. The fabrication of polymer blends is a creative method for the production of tailor-made materials for advanced applications that are able to combine functionalities from both components. In this study, poly(alkylene furanoate)/poly(alkylene terephthalate) blends with different compositions were prepared by solution blending in a mixture of trifluoroacetic acid and chloroform. Three different types of blends were initially prepared, namely, poly(ethylene furanoate)/poly(ethylene terephthalate) (PEF/PET), poly(propylene furanoate)/poly(propylene terephthalate) (PPF/PPT), and poly(1,4-cyclohenedimethylene furanoate)/poly(1,4-cycloxehane terephthalate) (PCHDMF/PCHDMT). These blends’ miscibility characteristics were evaluated by examining the glass transition temperature of each blend. Moreover, reactive blending was utilized for the enhancement of miscibility and dynamic homogeneity and the formation of copolymers through transesterification reactions at high temperatures. PEF–PET and PPF–PPT blends formed a copolymer at relatively low reactive blending times. Finally, poly(ethylene terephthalate-co-ethylene furanoate) (PETF) random copolymers were successfully introduced as compatibilizers for the PEF/PET immiscible blends, which resulted in enhanced miscibility.
[10] Z. Terzopoulou, E. Tarani, N. Kasmi, L. Papadopoulos, K. Chrissafis*, D.G. Papageorgiou, G.Z. Papageorgiou, D.N. Bikiaris*. Thermal Decomposition Kinetics and Mechanism of In-Situ Prepared Bio-Based Poly(propylene 2,5-furan dicarboxylate)/ Graphene Nanocomposites. Molecules. 2019, 24(9), 1717. LINK (IF “2021”:4.927)

Abstract: Bio-based polyesters are a new class of materials that are expected to replace their fossil-based homologues in the near future. In this work, poly(propylene 2,5-furandicarboxylate) (PPF) nanocomposites with graphene nanoplatelets were prepared via the in-situ melt polycondensation method. The chemical structure of the resulting polymers was confirmed by 1H-NMR spectroscopy. Thermal stability, decomposition kinetics and the decomposition mechanism of the PPF nanocomposites were studied in detail. According to thermogravimetric analysis results, graphene nanoplatelets did nοt affect the thermal stability of PPF at levels of 0.5, 1.0 and 2.5 wt.%, but caused a slight increase in the activation energy values. Pyrolysis combined with gas chromatography and mass spectroscopy revealed that the decomposition mechanism of the polymer was not altered by the presence of graphene nanoplatelets but the extent of secondary homolytic degradation reactions was increased.
[9] N. Kasmi, M. Majdoub, G.Z. Papageorgiou*, D.N. Bikiaris*. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate), Polymer Degradation and Stability. 2018, 152,177-190. LINK (This article belongs to the Special Issue Bio-based and biodegradable polymers and composites (BIOPOL 2017)) (IF “2021”: 5.204)

Abstract: In this paper, a series of fully biobased poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate)s (PCIsFs) copolyesters have been synthesized from dimethyl-2,5-furandicarboxylate (DMFD), isosorbide (IS) and 1,4-cyclohexanedimethanol (CHDM) by melt polycondensation. The structure of prepared polymers was characterized by 1H NMR and FTIR spectroscopies. Differential scanning calorimetry (DSC) results indicated that the crystallizability and melting temperature (Tm) of PCIsFs can be controlled with the increasing content of isosorbide furanoate units into the copolyesters structure, whereas their glass transition temperatures (Tg) showed a beneficial increase ranging from 76.8 °C to 103.5 °C. Wide-Angle X-ray Diffractometry (WAXD) patterns revealed that a small portion of isosorbide units are inserted into the crystals of PCHDMF, resulting in increase of the interplanar distances and crystal defects. A banded spherulitic morphology was observed, while coarsening of spherulites and increase in band distance occurred with increasing isosorbide content. Thermogravimetric analysis (TGA) results showed an excellent thermal stability up to 360 °C for all copolymers.
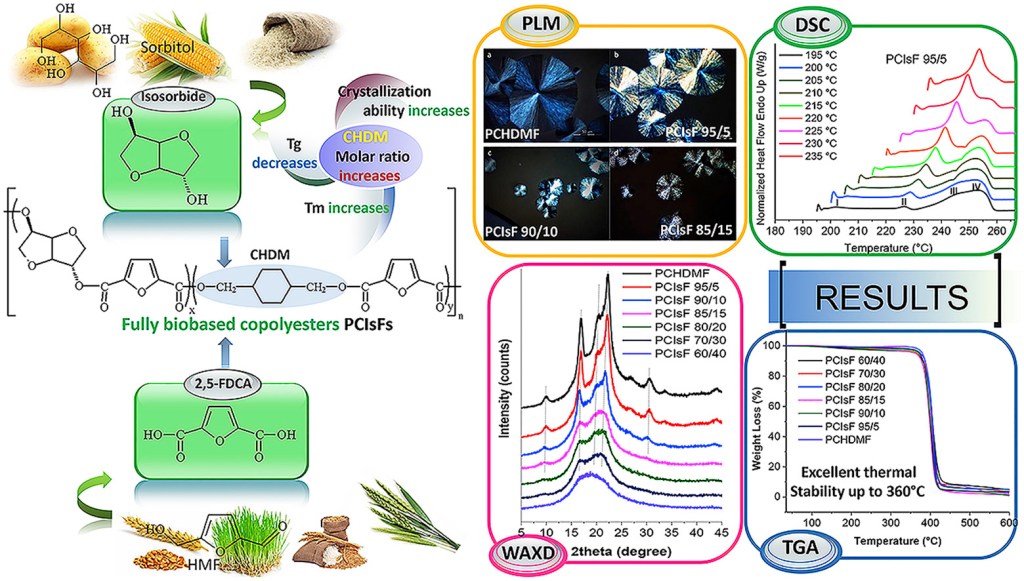
[8] N. Kasmi, G.Z. Papageorgiou*, D.S. Achilias, D.N. Bikiaris*. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications, Polymers. 2018, 10, 471. LINK (This article belongs to the Special Issue Polymers from Renewable Resources) (IF “2021”: 4.967)

Abstract: The goal of this study was to synthesize, through a facile strategy, high molecular weight poly(ethylene furanoate) (PEF), which could be applicable in food packaging applications. The efficient method to generate PEF with high molecular weight consists of carrying out a first solid-state polycondensation under vacuum for 6 h reaction time at 205 °C for the resulting polymer from two-step melt polycondensation process, which is catalyzed by tetrabutyl titanate (TBT). A remelting step was thereafter applied for 15 min at 250 °C for the obtained polyester. Thus, the PEF sample was ground into powder, and was then crystallized for 6 h at 170 °C. This polyester is then submitted to a second solid-state polycondensation (SSP) carried out at different reaction times (1, 2, 3.5, and 5 h) and temperatures 190, 200, and 205 °C, under vacuum. Ultimately, a significant increase in intrinsic viscosity is observed with only 5 h reaction time at 205 °C during the second SSP being needed to obtain very high molecular weight PEF polymer greater than 1 dL/g, which sufficient for manufacturing purposes. Intrinsic viscosity (IV), carboxyl end-group content (–COOH), and thermal properties, via differential scanning calorimetry (DSC), were measured for all resultant polyesters. Thanks to the post-polymerization process, DSC results showed that the melting temperatures of the prepared PEF samples were steadily enhanced in an obvious way as a function of reaction time and temperature increase. It was revealed, as was expected for all SSP samples, that the intrinsic viscosity and the average molecular weight of PEF polyester increased with increasing SSP time and temperature, whereas the number of carboxyl end-group concentration was decreased. A simple kinetic model was also developed and used to predict the time evolution of polyesters IV, as well as the carboxyl and hydroxyl end-groups of PEF during the SSP.
[7] N. Kasmi, Z. Terzopoulou, G.Z. Papageorgiou, D.N. Bikiaris*. Poly(1,4-cyclohexanedimethylene 2,6-naphthalate) polyester with high melting point: effect of different synthesis methods on molecular weight and properties, eXPRESS Polymer Letters. 2018, 12, 227-237. LINK (IF “2020”: 4.161)

Abstract: In the current manuscript, a new approach for the synthesis of poly(1,4- cyclohexanedimethylene 2,6-naphthalate) (PCHDMN) derived from dimethyl 2,6-naphthalenedicarboxylate (2,6-DMN) and 1,4-Cyclohexanedimethanol (CHDM) via melt polycondensation method is introduced. The effect of three different synthesis pathways, polycondensation time and temperature on polyesters molecular weight increase has been investigated. All of the prepared samples were characterized measuring their intrinsic viscosity (IV), thermal properties and morphology with differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD), respectively. The results demonstrated the effectiveness of the synthesis pathway proposed for the preparation of PCHDMN, resulting in high molecular weight (IV value around 0.5 dL/g) and much shorter reaction time. Melt polycondensation temperatures above melting point of polyester should be avoided to be used due to the decomposition of polyester. This was proved by thermogravimetric analysis (TGA) and Pyrolysis-gas chromatography–mass spectroscopy analysis (Py-GC/MS).
[6] N. Poulopoulou, N. Kasmi, D.N. Bikiaris, D.G. Papageorgiou, G. Floudas, G.Z. Papageorgiou*. Sustainable polymers from renewable resources: Polymer blends of furan-based polyesters, Macromolecular Materials and Engineering. 2018, 1800153. LINK (IF “2021”: 4.402)

Abstract: A series of blends of furan‐based green polyesters, for eco‐friendly packaging materials, are synthesized. Poly(ethylene 2,5‐furandicarboxylate) (PEF), poly(propylene 2,5‐furandicarboxylate) (PPF), and poly(butylene 2,5‐furandicarboxylate) (PBF) are synthesized by applying melt polycondensation. Blends of the above polyesters with 50/50 w/w composition as well as blends of furanoate/terephthalate (PPF/PPT) are also prepared. The glass temperature along with the crystallization and melting behaviors of melt quenched blends are studied aiming at understanding their dynamic state and miscibility. Based on their Tg and crystallization behavior, PEF/PPF shows dynamic homogeneity and miscibility whereas PPF/PBF and PEF/PBF exhibit partial miscibility and immiscibility, respectively. In an effort to dynamically homogenize the compounds, reactive blending is applied and the behavior of the resulting blends is monitored following quenching. A profound improvement in blend homogenization is observed with increasing melt mixing time for the PPF/PPT sample, evidenced by the single glass temperature and by the narrowing in liquid‐to‐glass regime. The obtained single glass temperature together with the suppressed tendency for crystallization with increasing mixing time are taken as evidences of dynamic and thermodynamic homogeneity.
[5] N. Kasmi, M. Majdoub, G.Z. Papageorgiou*, D.S. Achilias, D.N. Bikiaris*. Solid-state polymerization of poly(ethylene furanoate) biobased polyester, I: Effect of catalyst type on molecular weight increase, Polymers. 2017, 9, 607. LINK (This article belongs to the Special Issue Biodegradable and Biobased Polyesters) (IF “2021”: 4.967)

Abstract: In this work, we report the synthesis of poly(ethylene furanoate) (PEF), catalyzed by three different catalysts, namely, titanium (IV) isopropoxide (TIS), tetrabutyltitanate (TBT), and dibutyltin (IV) oxide (DBTO), via the two-stage melt polycondensation method. Solid-state polymerization (SSP) was conducted at different reaction times (1, 2, 3.5, and 5 h) and temperatures 190, 200, and 205 °C, under vacuum. The resultant polymers were analyzed according to their intrinsic viscosity (IV), end groups (–COOH), and thermal properties, via differential scanning calorimetry. DSC results showed that the post polymerization process was favorable to enhance the melting point of the prepared PEF samples. As was expected, the intrinsic viscosity and the average molecular weight of PEF increased with the SSP time and temperature, whereas the number of carboxyl end-groups was decreased. A simple kinetic model was also developed and used to predict the time evolution of polymers IV, as well as the carboxyl and hydroxyl content of PEF during the SSP. From both the experimental measurements and the theoretical simulation results it was proved that the presence of the TIS catalyst resulted in higher transesterification kinetic rate constants and higher reaction rates. The activation energies were not much affected by the presence of different catalysts. Finally, using DBTO as a catalyst, the polyesters produced have higher crystallinity, and as a consequence, higher number of inactive carboxyl and hydroxyl groups.
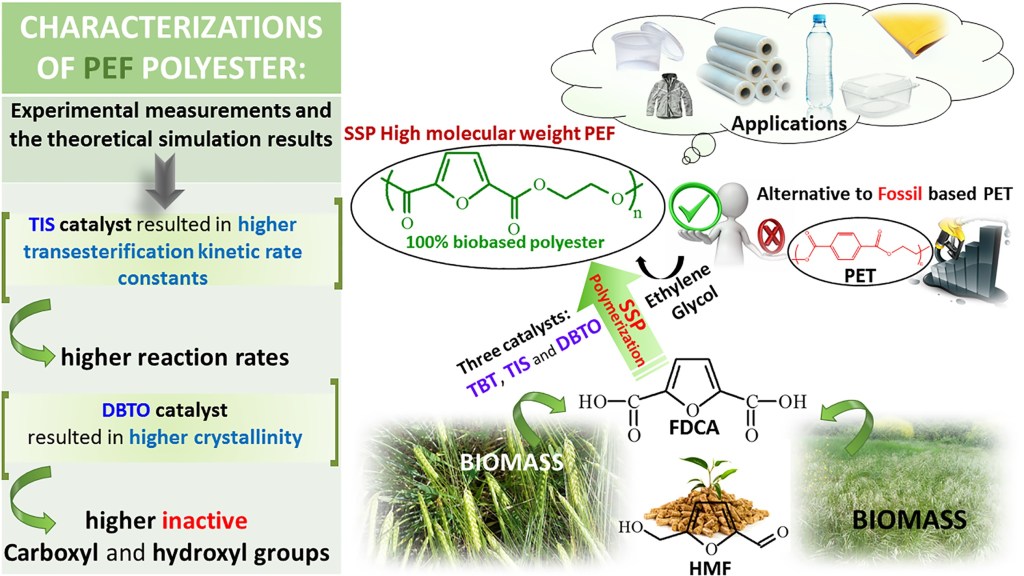
[4] N. Kasmi, M. Roso, N. Hammami, M. Majdoub, C. Boaretti, P. Sgarbossa, C. Vianello, G. Maschio, M. Modesti, A. Lorenzetti*. Microwave-assisted synthesis of isosorbide-derived diols for the preparation of thermally stable thermoplastic polyurethane, Designed Monomers and Polymers. 2017, 20, 547-563. LINK (CiteScore “2019”: 3.7)

Abstract: In order to prepare thermally stable isosorbide-derived thermoplastic polyurethane, the synthesis of two new chiral exo–exo configured diols, prepared from isosorbide, and two types of diphenols (bisphenol A and thiodiphenol) was described. The synthesis conditions were optimized under conventional heating and microwave irradiations. To prove their suitability in polymerization, these monomers were successfully polymerized using 4,4′-diphenylmethane diisocyanate (MDI) and hexamethylene diisocyanate (HDI). Both monomers and polymers have been studied by NMR, FT-IR, TGA, DSC; intrinsic viscosity of polymers has also been determined. The results showed the effectiveness of the synthetic strategy proposed; moreover, a dramatic reduction of the reaction time and an important improvement of the monomers yield using microwave irradiation have been demonstrated. The monomers, as well as the polymers, showed excellent thermal stability both in air and nitrogen. It was also shown that the introduction of sulphur in the polyurethane backbone was effective in delaying the onset of degradation as well as the degradation rate.

[3] Z. Terzopoulou, N. Kasmi, V. Tsanaktsis, N. Doulakas, D.N. Bikiaris*, D.S. Achilias, G.Z. Papageorgiou*. Synthesis and Characterization of Bio-Based Polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexane dimethylene-2,5-furanoate), Materials. 2017, 10, 801. LINK (This article belongs to the Special Issue Biobased Polymers for Packaging Applications) (IF “2021”: 3.748)

Abstract: In the present study, three new biobased furanoate polyesters with potential use in food packaging applications, named poly(isosorbide furanoate) (PIsF), poly(methyl-propylene furanoate) (PMePF) and poly(1,4-cyclohexane-dimethylene 2,5-furanoate) (PCHDMF) were synthesized. As monomers for the preparation of the polyesters, 2,5-furandicarboxylic acid (FDCA) and diols with irregular or complicated structure were used, including isosorbide (IS), 2-methyl-1,3-propanediol (MPD) and 1,4-cyclohexane-dimethanol (CHDM). The polymerization process was carried out via melt polycondensation method. The structural characteristics and thermal behavior of the polymers were studied. The kinetic fragility of the amorphous phase of the polymers was evaluated. The thermal degradation was studied by means of thermogravimetry and a pyrolysis Py-GC/MS (Pyrolysis-Gas Chromatography/Mass Spectroscopy) system to estimate the degradation mechanism.
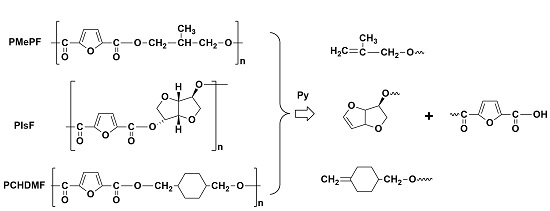
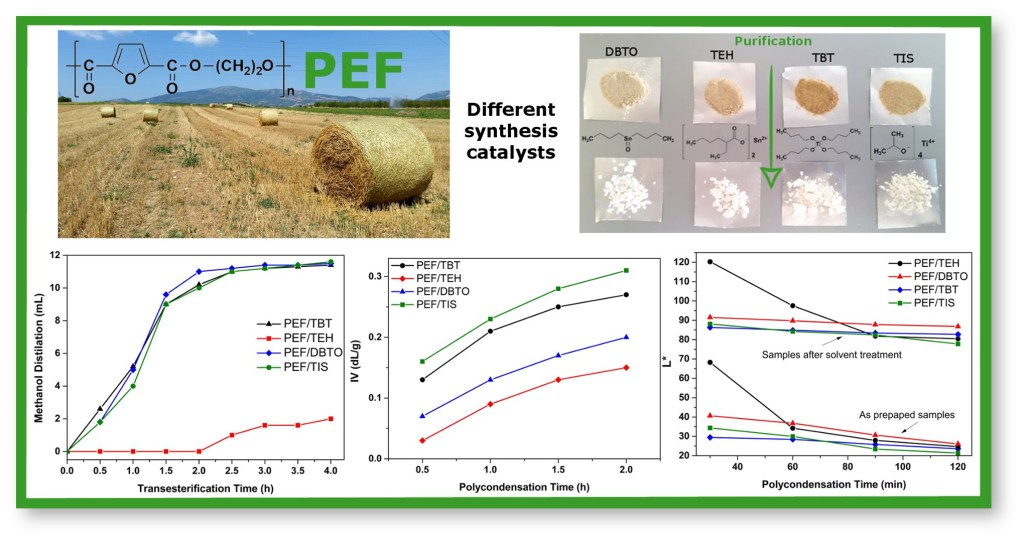
[2] Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, V. Tsanaktsis, N. Nikolaidis, M. Kostoglou, G.Z. Papageorgiou, D.A. Lambropoulou, D.N. Bikiaris*. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation, Polymer Chemistry. 2017, 8, 6895-6908. LINK (IF “2021”: 5.364)
Abstract: In this work, the effect of the catalysts tetrabutyl titanate(IV) (TBT), titanium(IV) isopropoxide (TIS), tin(II) 2-ethylhexanoate (TEH) and dibutyltin(IV) oxide (DBTO) on the synthesis of poly(ethylene furanoate) (PEF) was studied during a two-stage melt polycondensation process. In all reactions, 2,5-dimethylfuran-dicarboxylate (DMFD) and ethylene glycol (EG) in 1 : 2 molar ratios, and 400 ppm of catalyst were used. The rate of the transesterification reaction (first stage) was evaluated by measuring the volume of the distilled methanol and for the polycondensation reaction (second stage) by the increase of intrinsic viscosity. For the first stage, all catalysts had a similar effect to methanol distillation, except for TEH which was found to be the slowest catalyst, while for the second stage TIS and TBT were found to be the most effective catalysts, followed by DBTO and TEH, which again had the lowest reactivity. Coloration of the prepared polyesters was measured using the L*a*b* colour space system and was found to be dependent on catalyst type and melt polycondensation time, with titanate catalysts yielding the highest coloration. White coloured polyesters can be obtained after dissolution in trifluroacetic acid and chloroform mixture, and precipitation in methanol. Decomposition by-products formed throughout the different processes were identified in solution and elucidated by using liquid chromatography high resolution mass spectrometry (LC-HRMS). Similar decomposition products were detected in all chromatographs and therefore concentration in samples prepared with titanate catalysts might be the cause of the higher colour intensity of these samples.
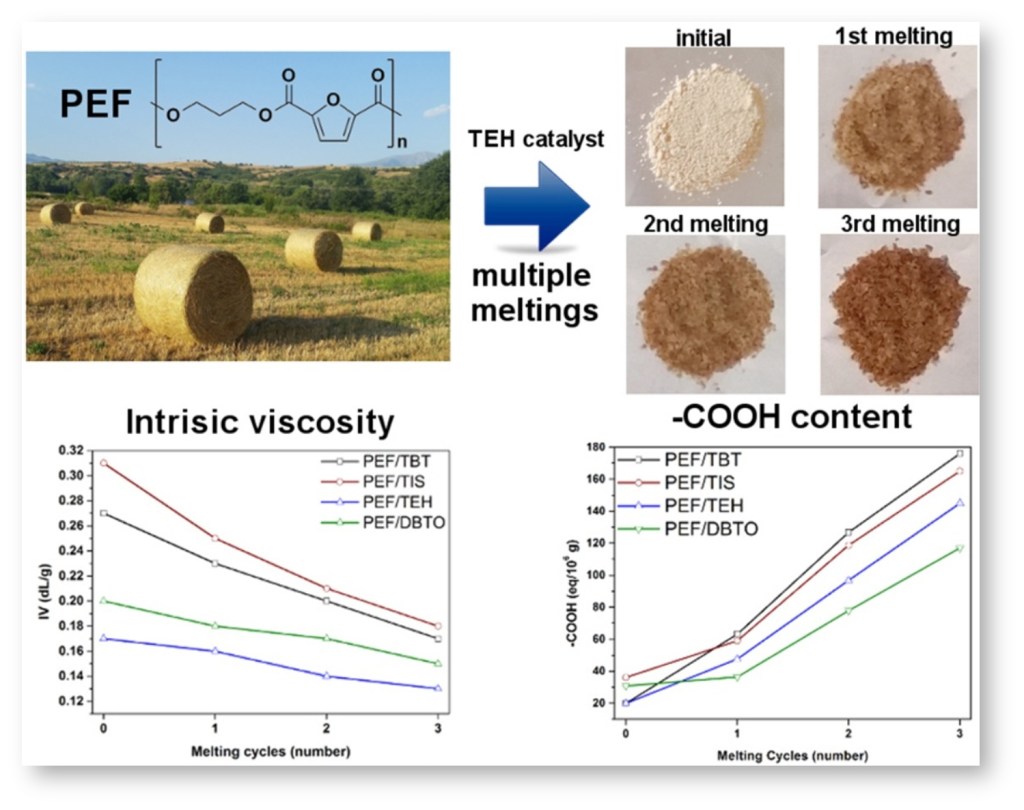
[1] Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, M. Majdoub, G.Z. Papageorgiou, D.N. Bikiaris*. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester, Journal of Analytical and Applied Pyrolysis. 2017, 126, 357-370. LINK (IF “2021”: 6.437)

Abstract: In the present work, poly(ethylene furanoate) (PEF) was synthesized via two-stage melt polycondensation process, using four different catalysts, namely tetrabutyl titanate (TBT), titanium(IV) isopropoxide (TIS), tin(II) 2-ethylhexanoate (TEH) and dibutyltin(IV) oxide (DBTO). In all reactions, 2,5-dimethylfuran-dicarboxylate (DMFD), ethylene glycol (EG) (1:2 molar ratio) and 400 ppm of catalyst were used. The effect of these catalysts on the remelting and decomposition mechanism of PEF was studied. All polyesters were remelted up to three times, and for every melting cycle the intrinsic viscosity (IV) values and the carboxyl end group content were measured. It was found that after every melting, the IV was reduced while the COOH number was increased. Also, all samples have been studied with Fourier Transformed-Infrared Spectroscopy (FTIR), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) techniques. It was found that the molecular weight reduction also affects the thermal properties of the polyesters. The decomposition mechanism of the prepared polyesters was studied using pyrolysis-gas chromatography/mass spectroscopy (Py-GC/MS).